-
由于产生的废水量大且成分复杂,纺织行业被普遍认为是污染最严重的行业之一。据不完全统计,纺织行业每年生产超过
$ 1\times {10}^{6} $ t染料,其中60%~70%是偶氮化合物,其特点是含有一种或多种偶氮键(—N=N—)及各种衍生物[1]。由于其毒性、致癌性和致突变性,偶氮染料及其降解中间产物对水生生物的生长具有较强的抑制作用,排放到自然环境中,会导致水体植物和动物大量死亡。此外,硫化物通常存在于染料废水中,一方面是添加硫化钠还原偶氮化合物而形成,另一方面可能是其他广泛存在于纺织染色工艺中的硫物质转化而来,如硫酸盐、亚硫酸盐和硫代硫酸盐[2]。有研究[3]表明,大量的硫化钠用于硫化染料的染色过程(>90%),产生含由15%~20%硫化物的废水。由于其毒性、风化性和腐蚀性强,硫化物(水性硫化物和气体硫化物)会对人体健康产生严重危害。因此,在含硫化物的偶氮染料废水最终排入天然水体之前,必须对其进行有效的处理。微生物燃料电池(microbial fuel cells, MFC)是一种在微生物的催化代谢作用下,使有机/无机污染物发生氧化反应,释放质子和电子,从而产生电流、并降解污染物的装置[4]。在MFC的运行中,阳极液pH能影响底物的代谢活性,进而影响质子和电子的生成和转移机制,对MFC的整体性能有着重要作用。外部pH的变化可导致几种主要生理参数的变化,包括内部pH、离子浓度、膜电位和质子动力[5]。一般来说,细菌的最佳生长需要接近中性的pH,而阴极电极上的氧还原反应会导致pH呈碱性[6]。传统的双室MFC可以保持2种不同的pH环境,以优化阳极反应和阴极反应。然而,空气阴极MFC中,在只有一种电解质存在的条件下不能做到这一点。在现有的研究中,电解质pH对空气阴极MFC产电性能和污染物降解的相关研究较少,因此,研究空气阴极MFC的最佳pH以及电解质pH对产电性能的影响是必要的。
本研究构建了单室空气阴极MFC,且将其用于处理含硫偶氮染料废水,以乙酸钠为底物,碳刷和碳布分别作为反应器的阳极和阴极材料。探究了阳极液在不同初始pH条件下单室微生物燃料电池的产电性能及其对硫化物和偶氮染料的降解效果,确定了该反应器运行的最佳pH,同时探究了硫和染料的协同降解机理。鉴于染料出水中含有大量的硫化物,MFC中硫化物作为本体还原剂和电子供体,可以加速偶氮染料的降解。本研究为印染厂染料废水的降解提供了一个新的思路。
全文HTML
-
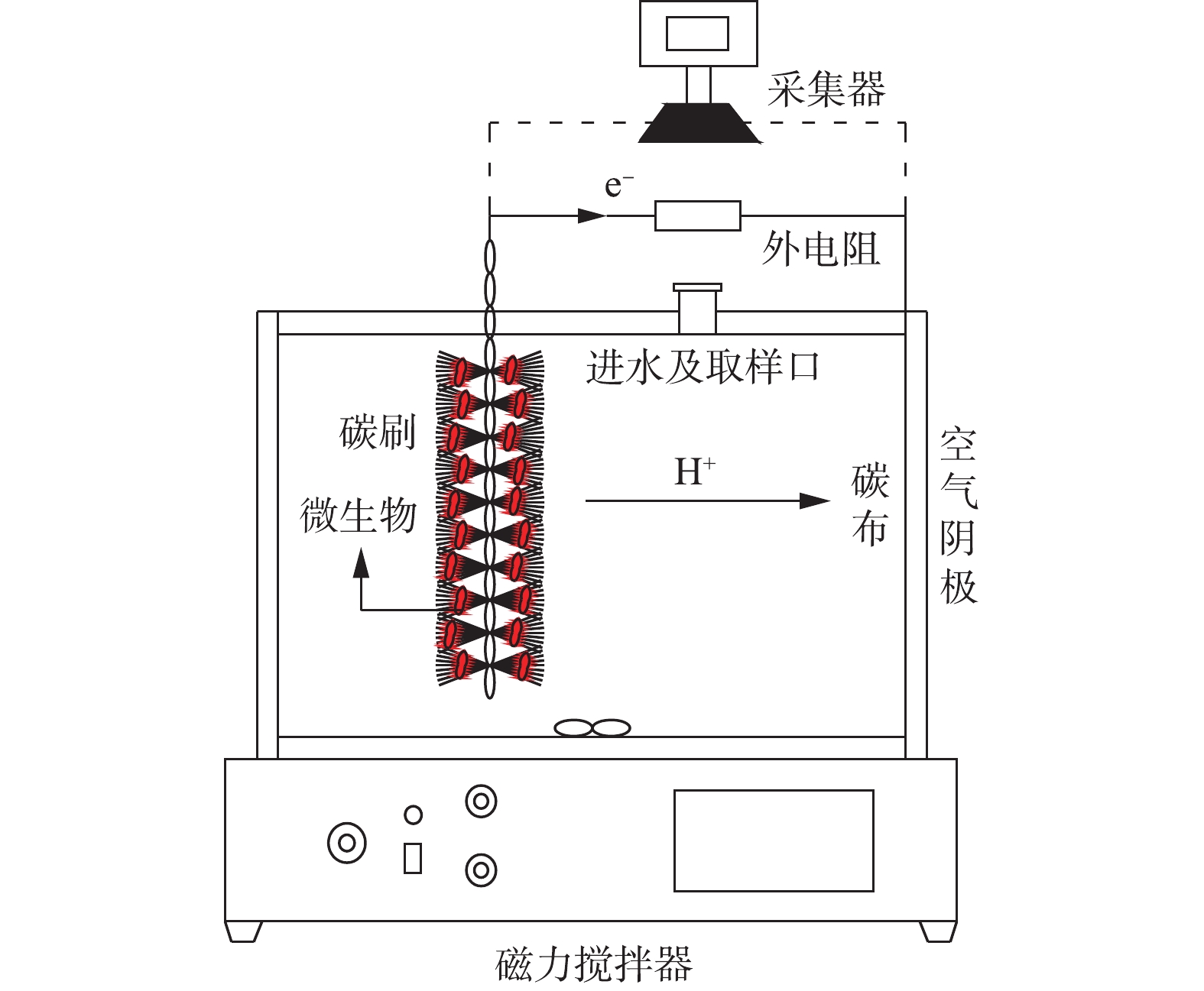
本研究采用单室空气阴极MFC,是在LOGAN典型的立方体型MFC结构基础上优化而成。将MFC的总圆柱形腔体积增加到400 mL,腔室的容积尺寸为φ16 cm×8 cm,示意图如图1所示。阳极电极为3 cm×6 cm石墨碳纤维刷(日本东丽),由钛丝和碳纤维丝组成。使用前,碳刷分别用1 mol·L−1 NaOH和1 mg·L−1 HCl浸泡24 h,再用去离子水浸泡12 h,目的是去除碳刷上多余的杂质,随后放置于450 ℃的马弗炉中烘烤30 min,以增大碳刷的电极面积。阴极材料为w1s1005疏水导电碳布(台湾碳能),先将碳布裁剪成直径为8 cm的圆形尺寸,在去离子水中超声清洗烘干。在碳布的空气侧均匀涂抹4层60%的PTFE,放置空气中干燥5~10 min,干燥后涂层呈微白色;将涂好的碳布置于370 ℃马弗炉中15 min,取出冷却后,涂层呈亮黑色,重复以上处理方式4次。在碳布亲水层均匀涂抹含20%铂碳催化剂(0.5 mg·cm−2)、Nafion®溶液(6.67 μL·mg−1)、异丙醇(3.33 μL·mg−1)和去离子水(0.83 μL·mg−1)的混合物,在室温下风干24 h。阳极和阴极通过钛丝(直径1 cm)与1 000 Ω的外部电阻连接,形成闭合电路。反应器所处的环境温度为(28±5) ℃。
-
本研究的驯化污泥取自重庆市唐家沱污水处理厂。阳极腔室接种厌氧和好氧混合污泥,以增加MFC体系微生物的多样性。为了加快驯化速度,使电极材料上富集产电微生物,将接种污泥加入含硫偶氮染料废水中,放置在磁力搅拌器上搅拌,阳极碳纤维刷在放入反应器之前浸泡在接种污泥中24 h。为保证体系内生物量充足,将接种污泥与反应基质按照1∶1的比例混合后,再将其加入MFC反应器中进行启动,每隔36 h取出上清液,并重新加入新的基质进行驯化。同时,用中泰科研EM 9636数据采集器连接电脑实时记录系统的产电电压。经过一段时间的培养,系统连续3个周期的最大稳定电压相同则代表系统启动成功。待系统稳定运行30 d之后,调整进水染料的pH,观察MFC的产电性能和污染物的降解情况。
选取典型的偶氮染料刚果红作为目标物。模拟废水主要成分如下:2.75 g·L−1 Na2HPO4、4.97 g·L−1 NaH2PO4·H2O、0.31 g·L−1 NH4Cl、0.13 g·L−1 KCl、12.5 mL·L−1微量金属元素溶液和1.25 mL·L−1维生素溶液。微量金属元素配比为:1.5 mg·L−1 NTA(氨三乙酸)、3 mg·L−1 MgSO4、0.5 mg·L−1 MnSO4·H2O、1 mg·L−1 NaCl、0.1 mg·L−1 FeSO4·7H2O、0.1 mg·L−1 CaCl2·2H2O、0.1 mg·L−1 CoCl2·6H2O、0.13 mg·L−1 ZnCl2、0.01 mg·L−1 CuSO4·5H2O、0.01 mg·L−1 AlK(SO4)2·12H2O、0.01 mg·L−1 H3BO3、0.025 mg·L−1 Na2MoO4、0.024 mg·L−1 NiCl2·6H2O、0.025 mg·L−1 Na2WO4·2H2O。
-
MFC电极两端的电压通过连接在电脑上的数据采集器(中泰科研EM 9636B)每5 min进行采集记录1次,并由电脑进行自动记录和保存。电流I由欧姆定律计算得到,计算方法见式(1)。
式中:I为电流;U为外电阻两端的电压;R为电极两端外电阻。
功率密度根据式(2)进行计算。
式中:P为功率密度,mW·m−2;U为MFC电压,V;I为电流,A;A为反应器阴极的有效面积,cm2。
刚果红的浓度通过紫外分光光度计(日本岛津UV-3600)在496 nm处测量吸光度获得,降解率根据式(3)进行计算。
式中:DCR为刚果红降解率;C0为刚果红初始浓度,mg·L−1;Ci为第i次取样刚果红浓度,mg·L−1。
硫化物浓度采用国家标准分析方法GB/T 16489-1996亚甲基蓝分光光度法,COD采用国家标准分析方法HJ 828-2017重铬酸钾法测定。
实验中不同pH条件下的功率密度曲线和极化曲线采用变电组法测定。MFC开路运行2 h,电压达到稳定状态之后,依次改变外电阻阻值为9 000、6 000、3 000、1 000、700、500、400、300、200、100、70、50、30、20、10 Ω。通过数据采集器实时记录电极两端的电压,当检测到的电压波动幅度在5 mV以内时,代表MFC在此电阻下达到稳定状态,检测时间大概为3~5 min。随后以电流密度为横坐标,分别以功率密度和电压为纵坐标绘制功率密度曲线和极化曲线。
循环伏安扫描(cyclic voltammetry,CV)采用电化学工作站在三电极体系下测得。以阳极碳刷为工作电极、阴极碳布为对电极、Ag/AgCl参比电极。扫描速度为5 mV·s−1,扫描电压为−0.6~0.6 V。本研究中的所有电势均在标准氢电极(SHE)下进行测试。测试前,反应后的样品以4 000 r·min−1的速度离心10 min,获得的上清液用于等量乙酸乙酯提取代谢物,然后在室温下用无水硫酸钠干燥提取物,最后将所得晶体溶解于少量的高效液相色谱级甲醇中用于高效液相色谱和LC-MS分析。高效液相色谱分析使用C18(250 mm×4.6 mm)反相柱进行,流动相为0.3∶0.7(体积比)的乙醇和水,流速为1 mL·min−1;紫外检测器波长设置为236 nm。LC-MS用乙腈铵将乙腈调整到pH=5.9,然后以3 mL·min−1的流速作为流动相,在400 ℃下使用氮气作为干燥气体(10 L·min−1),电喷雾电离源为负离子模式。
1.1. 微生物燃料电池构建
1.2. 微生物燃料电池的接种与驯化
1.3. 分析与计算
-
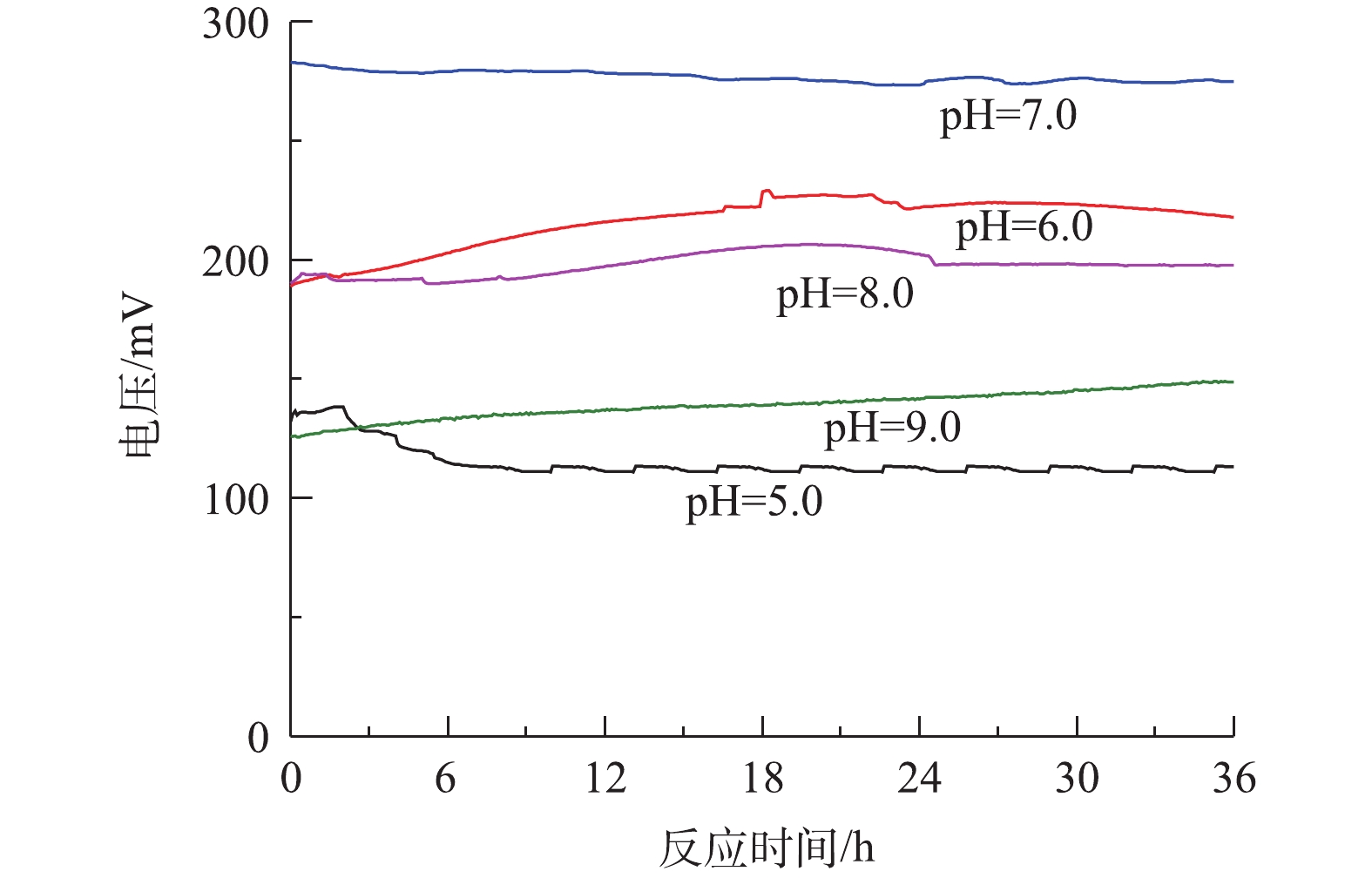
考察了MFC系统中pH对稳定电压的输出大小的影响,结果如图2所示。刚果红、硫化物和COD值分别固定为200、180和1 000 mg·L−1。在运行周期36 h内,不同pH条件下对应的输出电压数值波动幅度较小,表示MFC处于稳定的产电状态。由图2可知,pH对MFC的产电性能有较大的影响,当溶液初始pH=7.0、外电阻为1 000 Ω条件下,MFC最大的稳定输出电压为275.3 mV;当pH升高到8.0和9.0时,最大稳定输出电压分别降低至206.4 mV和140.3 mV;当pH降低为5.0和6.0时,最大稳定输出电压分别降低至113.5 mV和226.7 mV。pH=7.0时的稳定电压较pH=5.0时提高了58.77%,较pH=9.0时提高了49.04%,表明在中性条件下产电电压最高,其次是偏酸和偏碱条件,而在过酸和过碱条件下产电效果最差。其原因可能是,在中性条件下,阳极液中的产电菌活性最高,产甲烷菌的活性受到抑制,电子转移速率增加,有助于提高系统的输出电压;在偏酸或偏碱的环境下,阳极液中产电菌的活性受到抑制,电子输出减少,输出电压降低。因此,在含硫偶氮染料废水中的优势菌种适宜在中性环境下生长,pH=7.0有利于微生物的生长、繁殖和代谢,使得中性条件下阳极生物膜上富集的产电微生物增加,胞外电子转移速率加快,单室空气阴极MFC的输出电压最高。
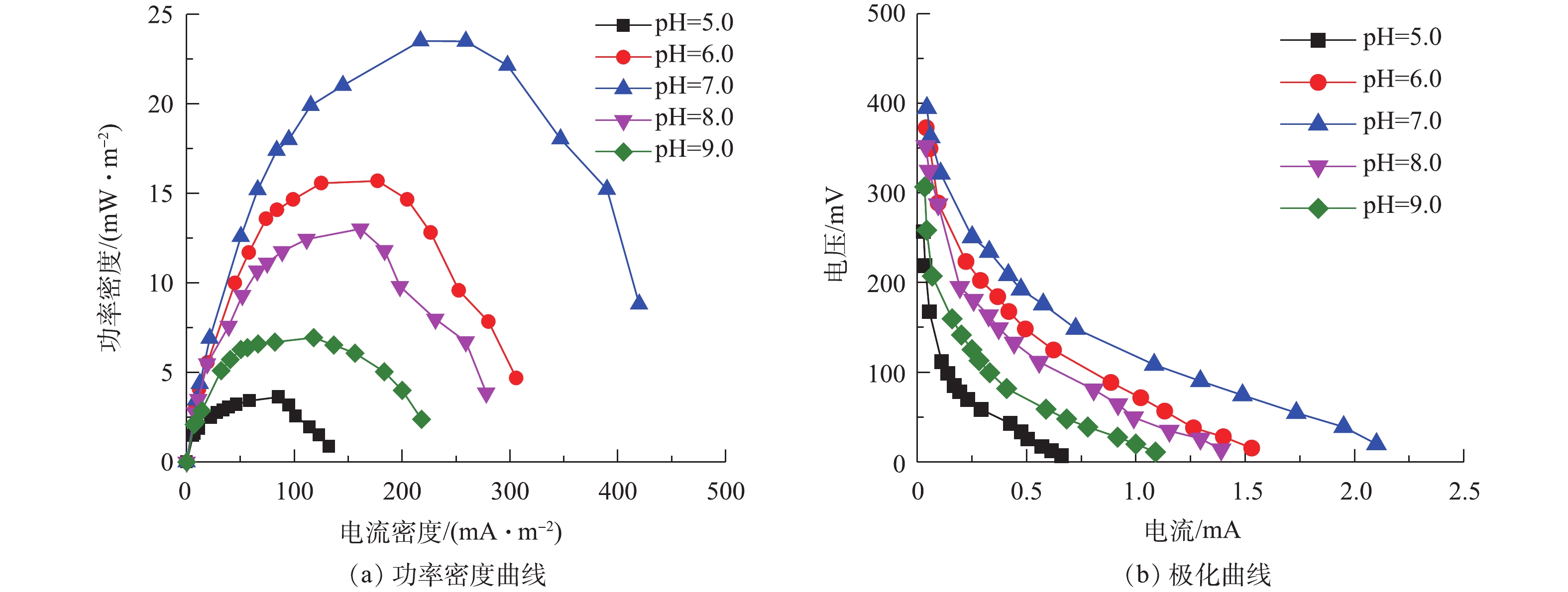
图3为不同pH条件下的功率密度曲线和极化曲线。当3个周期的驯化电压保持相对稳定(相差不超过20 mV)时,表示MFC驯化成功。从第4个周期开始,采用外电阻梯度变化方法,分别测定不同pH条件下MFC的功率密度曲线和极化曲线,结果如图3(a)所示。可以看出,最大功率密度分别为3.63 (pH=5.0)、15.69 (pH=6.0)、23.50 (pH=7.0)、13.02 (pH=8.0)和6.93 mW·m−2 (pH=9.0)。在中性条件下功率密度最大,其次为偏酸和偏碱条件,过酸和过碱条件下功率密度最小。在pH=7.0时系统的功率密度最大,较pH=5.0时提高了84.55%,较pH=9.0时提高了70.51%,这说明阳极液pH过高或过低均会抑制产电菌的活性,影响MFC阳极反应速率,减低体系的最大功率密度,从而影响系统的整体产电性能。在pH=7.0时,含硫偶氮染料废水MFC的最大功率密度为23.50 mW·m−2,而SUN等[7]在单基质MFC中去除硫化物的最大功率密度为13 mW·m−2。这表明染料和硫化物均可以被微生物有效地利用,共同促进MFC的产电,也表明以偶氮染料作为电子受体的硫化物MFC有助于高功率输出。以上结果还表明,硫化物以及有机物和偶氮染料适合用作MFC中的电子供体和受体,含硫偶氮染料MFC是一种比较理想的燃料电池。
图3(b)为不同pH条件下,微生物燃料电池的极化曲线图。通过对极化曲线的线性拟合,可以得到在不同pH条件下的MFC内阻。5个电池(pH=5.0、6.0、7.0、8.0和9.0)的内阻分别为295.8、209.5、154.1、214.2和218.9 Ω。在反应器构型、材料和底物浓度等均相同的情况下,不同pH下MFC内阻表现出较大的差异,表明pH条件对微生物燃料电池的内阻有着较大的影响作用。在pH=7.0时,MFC的内阻最小,较pH=5.0时内阻降低了47.9%,表明中性条件有利于阳极产电微生物将生物化学能转化为电能,且系统内阻的降低似乎是产生更多电能的原因,在过酸或过碱性的条件下,阳极电位较高导致功率输出较低。在pH=7.0时,含硫偶氮染料废水中的阳极产电微生物活性较高,质子通过电解质的流动速度更快,故降低了内部阻力。5个电池的内阻均出现了活化极化区,欧姆极化区和浓差极化区,不同极化现象的存在使得电池的实际电压要远低于理论电压。因此,体系中的pH不仅影响阳极微生物的组成,也通过影响质子迁移而影响产电。GIL等[8]采用双室MFC装置研究发现,阳极基质的pH为7.0~8.0有利于MFC产电,与本研究的单室MFC所得的结论一致。
-
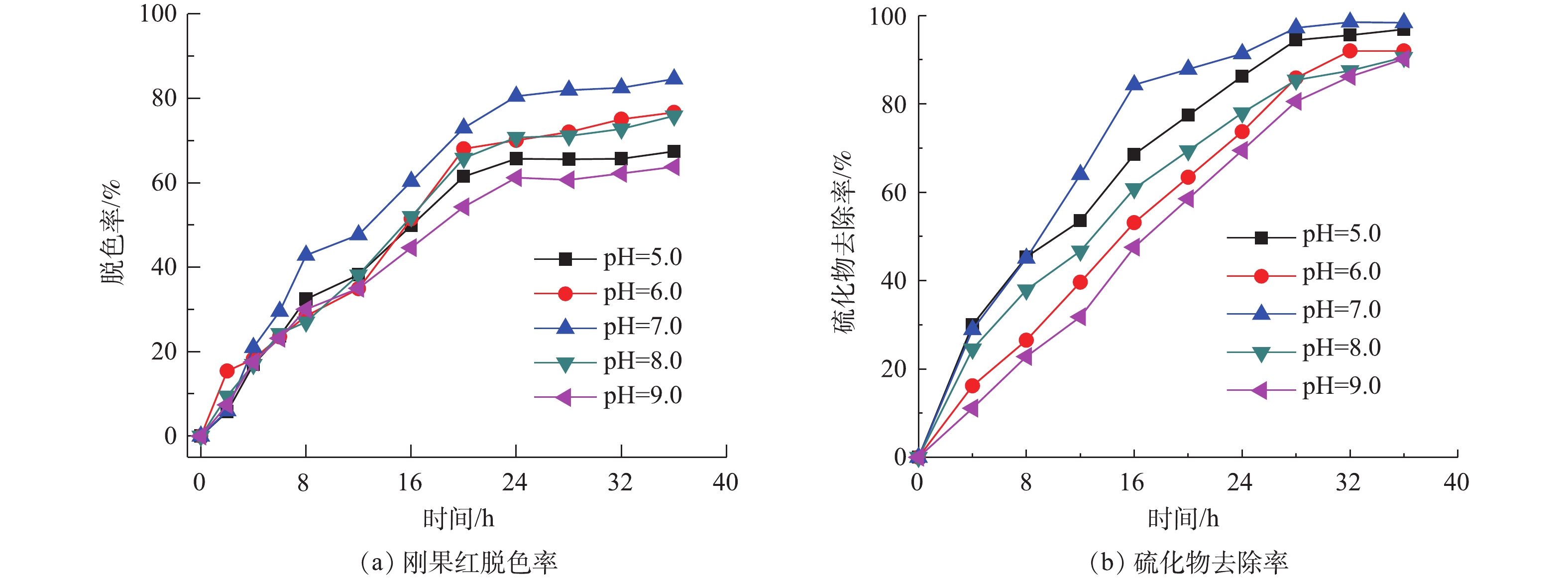
图4为不同pH条件下的刚果红脱色率和硫化物去除率变化情况。在大部分生物化学处理的设计和操作中,pH是至关重要的,pH影响着负责微生物活性的酶系统。因此,在废水处理中,pH对酶活性的影响进一步转化为对涉及到的相应微生物的影响。高于或者低于最佳pH均会导致酶活性的降低,进而影响反应速率。如图4(a)所示,在初始pH=7.0时,刚果红的脱色率最高为84.60%,在初始pH分别为5.0、6.0、8.0和9.0时,染料的脱色率分别为67.45%、76.66%、75.83%和63.78%。在中性条件下刚果红的脱色率最高,其次为偏酸或偏碱环境,而过酸或过碱环境下脱色率最低,说明过酸或过碱的环境抑制了微生物的代谢和生长,降低了酶的活性,从而降低了染料的降解效率。染料的脱色过程主要是通过氧化还原反应进行的,其中偶氮染料作为末端电子受体,在反应分解代谢过程中,MFC释放的电能主要取决于电子供体和电子受体之间的电位差。因此,为了能够最大限度地实现硫化物氧化和染料还原,还原电位较低的厌氧环境更加适合产电[9]。在中性条件下,硫的主要形式是的HS−,这就导致更负的阳极电势和来自MFC的更高电能[10]。因此,可以得出,中性条件有利于同时去除硫化物和偶氮染料,并有利于MFC发电。
如图4(b)所示,将初始pH分别设为5.0、6.0、7.0、8.0和9.0,外电阻为1 000 Ω,研究初始pH对硫化物氧化的影响。进水硫化物、染料浓度和COD分别维持在180、200和1 000 mg·L−1。在初始pH分别为5.0、6.0、7.0、8.0和9.0时,硫化物去除率分别为96.87%、91.98%、98.40%、88.53%和85.19%。在阳极液酸性和中性条件下可得到较高的硫化物去除率,表明中性和酸性条件更适合细菌生长,有利于硫酸盐的形成和硫化物的去除。当pH为5.0和9.0时,硫化物依然可以保持较高的降解率(96.87%和85.19%),表明MFC体系下的微生物具有较好的pH耐受性。MAHMOOD等[11]的研究表明,弱碱性条件有利于硫酸盐的形成。在碱性条件下,硫化物氧化可能导致过渡产物的积累,如硫代硫酸盐和多硫化物,其主要原因是,亚硫化氢离子的溶解性随着pH的升高而增加,其一旦溶解在水中,亚硫化氢就分解成硫化物离子,引起水的自电离,而高溶解的亚硫化氢离子可能抑制硫化物完全被氧化为硫酸盐,因此较高的pH会抑制硫化物的转化。对于大多数细菌来说,细菌生长的pH是4.0~9.0,而其最佳pH在6.5~7.5,一般来说,细菌更适宜于在弱碱性环境生存。然而,生物处理过程很少能完全在最佳生长条件下运行[12]。大多数先前报道的生物硫化物氧化系统均是在中性pH在7.0~8.0条件下运行的。
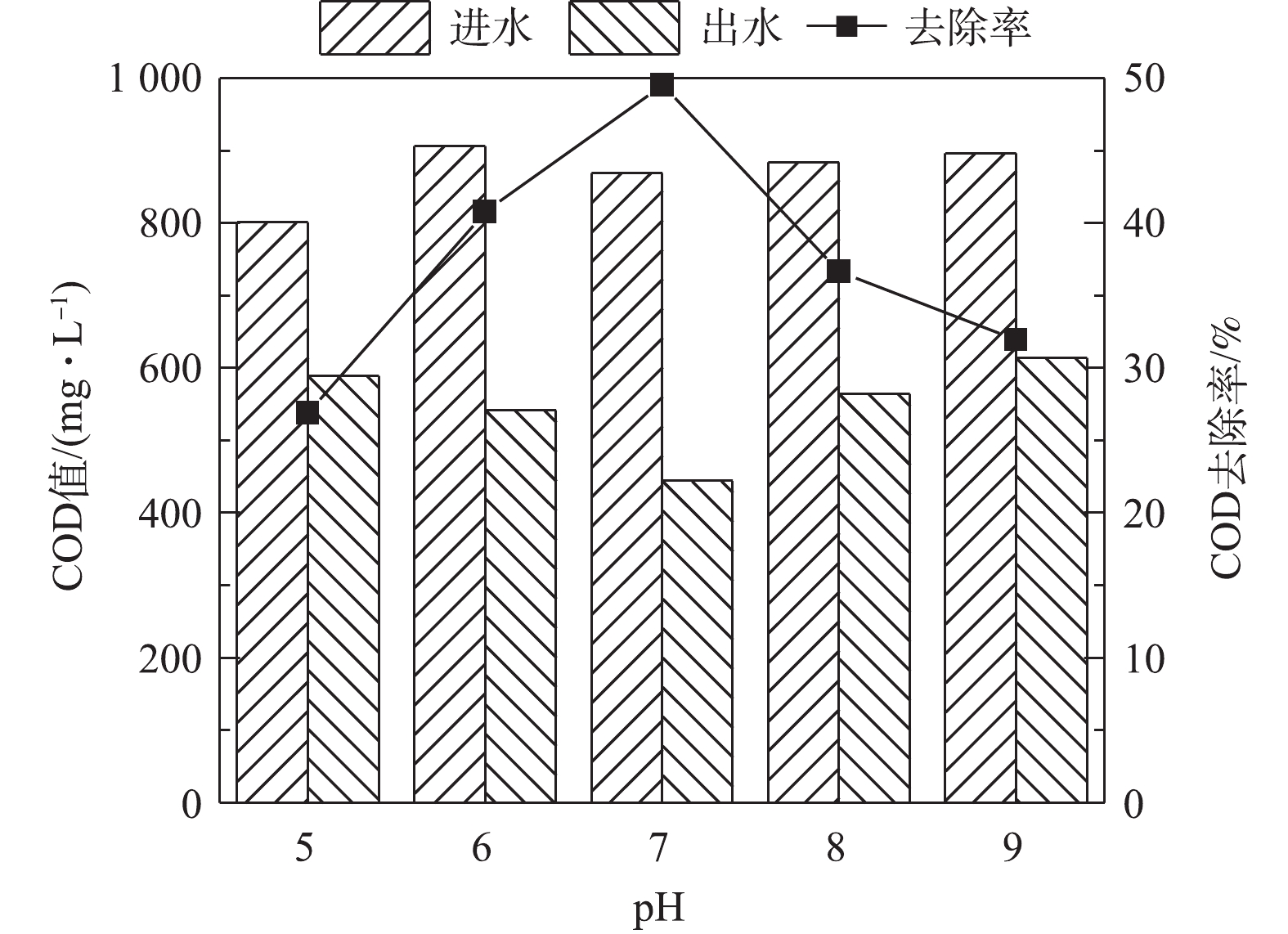
由图5可知,在中性pH的条件下,有机物的去除率最大,当pH降低至5.0时,COD去除率从49.56%下降到26.89%,当pH升高至9.0时,COD去除率下降到31.92%。单室MFC降解含硫偶氮染料废水中,COD的去除率不高,主要原因在于,硫化物和乙酸盐均为MFC体系中的电子供体,且硫化物的氧化电位较低,故先于乙酸盐发生氧化反应。在MFC中,乙酸盐作为复杂的大分子有机物并不会直接产生电能,而是被阳极产电菌分解为简单的小分子[13-14]。在微生物燃料电池的阳极膜上附着有大量的产电微生物,他们在细菌和电极的胞外电子转移过程中发挥着重大作用。而MFC中COD的去除是阳极微生物、产甲烷菌和发酵细菌的代谢活动综合作用的结果。当pH过酸或过碱影响了微生物的生长和代谢时,一方面大分子有机物转化为小分子有机物过程的速率降低;另一方面,阳极材料上产电细菌的功能也会受到抑制作用。因此,酸性和碱性条件下COD的去除率也会随之降低。
-
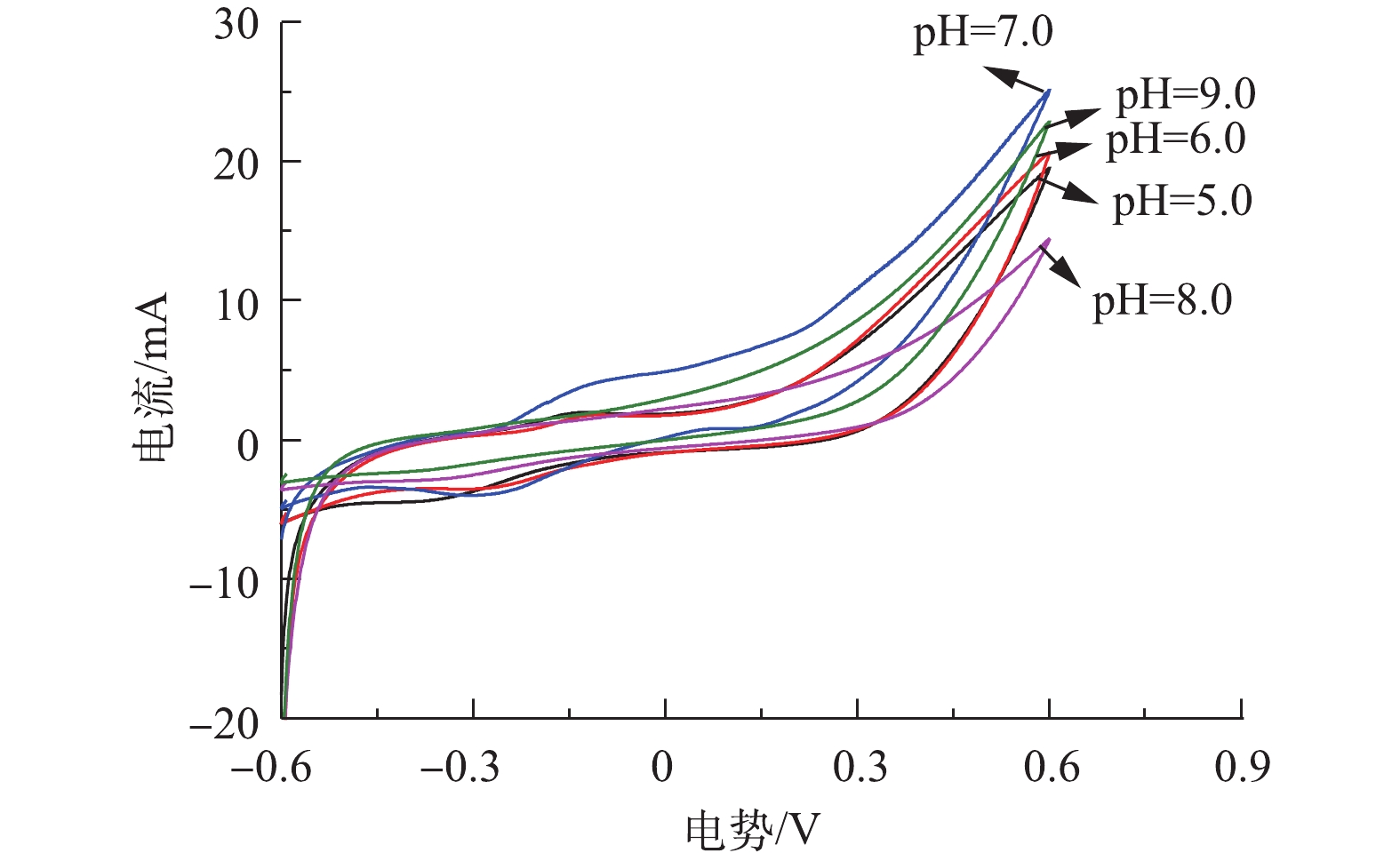
循环伏安法是一种常用的电化学研究方法,通过1次三角波扫描,完成1个还原和氧化反应的循环, 其电流-电压曲线就是循环伏安图,本研究通过循环伏安特性曲线来表征不同pH环境下阳极生物膜的氧化还原能力。由图6可知,当对其进行正向扫描时,在初始pH=7.0时,CV扫描曲线的氧化峰在−0.3 V时开始出现,表明此时电子从细菌转向电极,随后电流上升,表明生物膜上大量处于还原态的活性物质被氧化并形成氧化峰,而其他4个反应器没有明显的氧化峰。同时,5个反应器的还原峰相似,峰电流大小均在−0.005 A左右,表明pH条件的改变主要影响氧化反应速率。由图6可以看出,5个反应器的CV扫描形状比较接近,由CV曲线的闭合面积可知,当pH=5.0时闭合面积最小,当pH=7.0时闭合面积最大。CV曲线的闭合面积大小可以表示电极传输的电子数,并反映电极的电容。闭合面积越大,电极的电容就越好[15]。当pH=7.0时曲线面积最大,说明此时催化效率最高,电子转移速率最快。
-
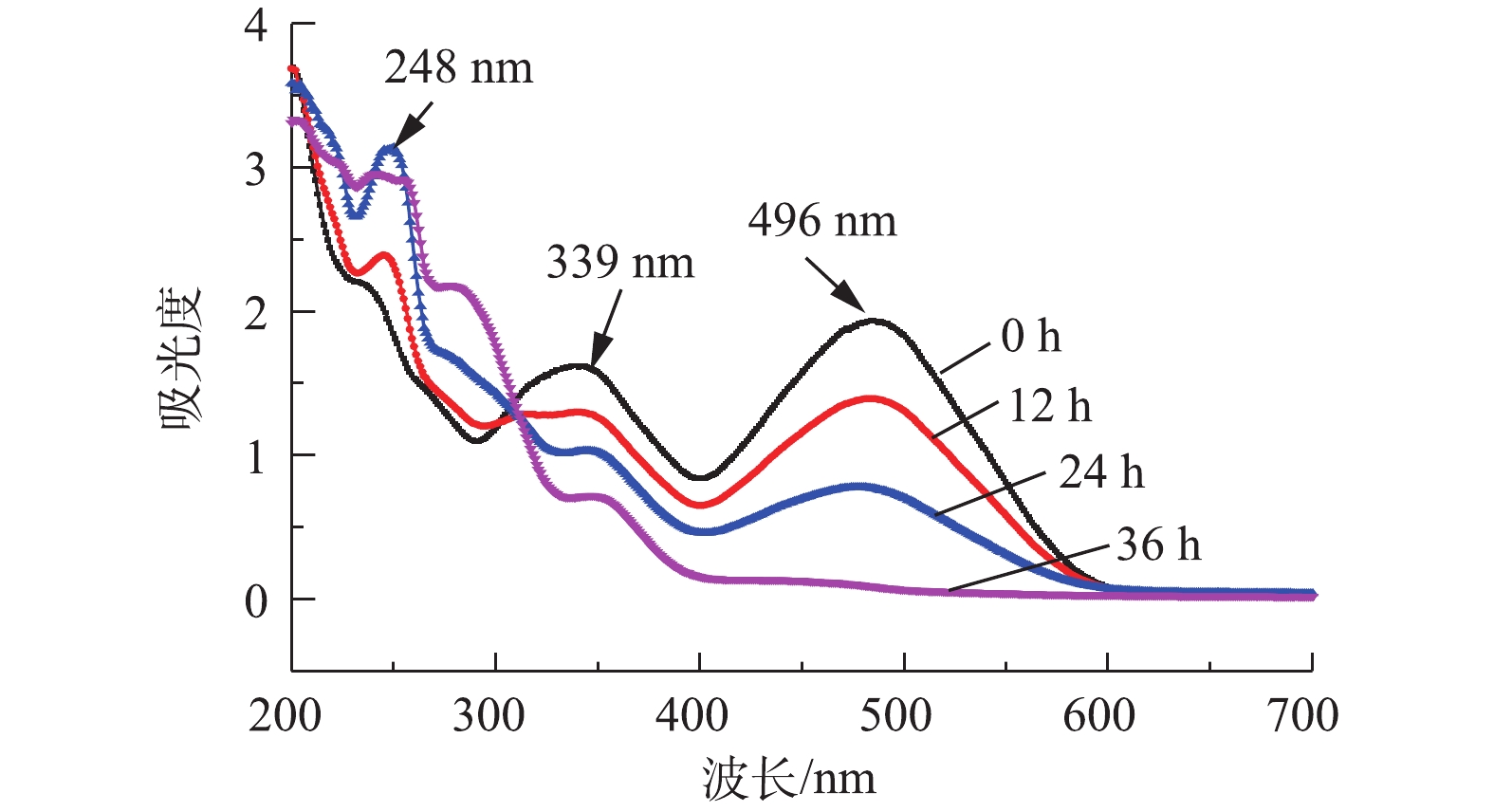
采用紫外-可见光扫描分光光度法测定了刚果红在最大吸收波长(λmax=496 nm)下的吸光度。偶氮键(—N=N—)与萘环和苯环形成的官能团在496 nm呈现吸收峰,使得刚果红染料的颜色呈红色[16],由图7可见,随着反应时间的增加,刚果红在496 nm处的吸收峰逐渐降低,在36 h后此处的吸收峰基本消失,表明含硫化物的偶氮染料在MFC中一个周期后基本反应完全。在36 h内,248 nm处出现明显吸收峰,表明有中间体生成,在24~36 h之间此处吸收峰未明显降低,表明中间体并未进一步降解。
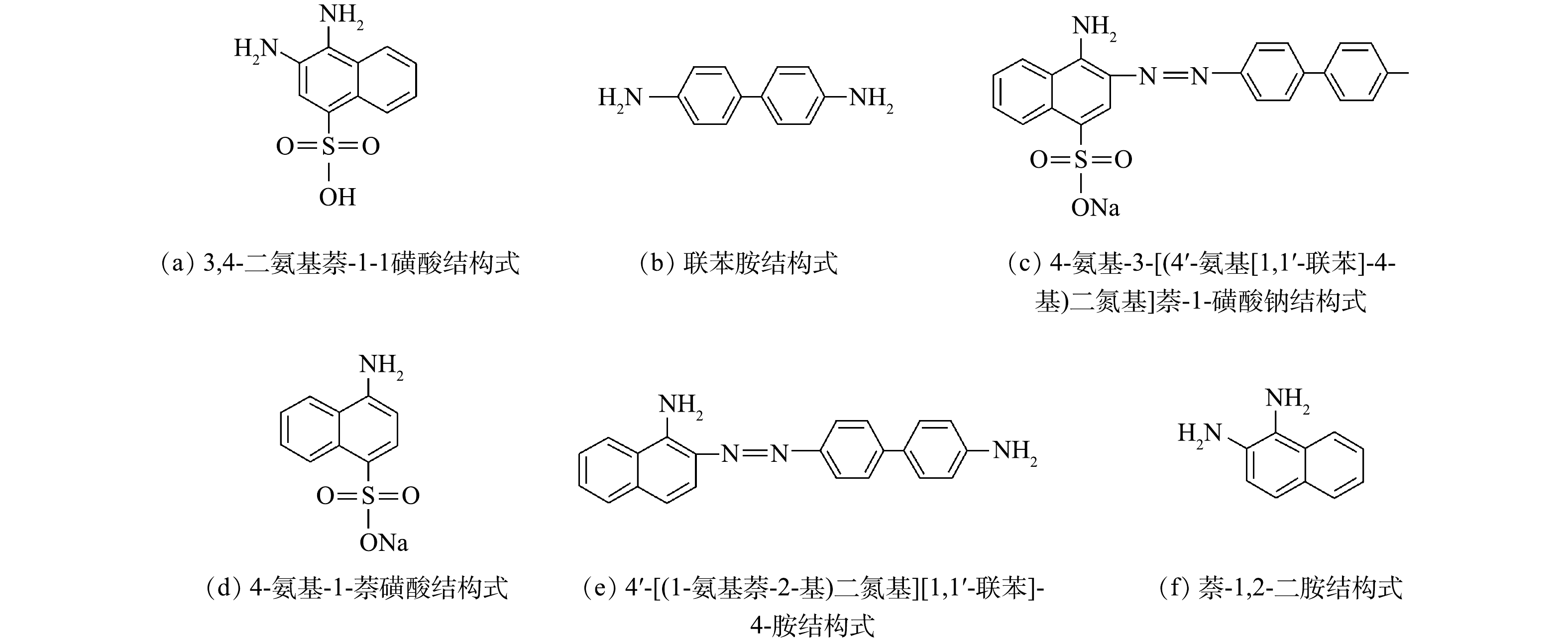
为深入分析刚果红中间产物,用液相色谱-质谱法对降解产物进行鉴定。如表1和图8所示,刚果红的还原产物可能是3,4-二氨基萘-1-磺酸、4-氨基-3-[(4'-氨基[1,1'-联苯]-4-基)二氮基]萘-1-磺酸钠、4-氨基-1-萘磺酸钠,4'-[(1-氨基萘-2-基)二氮基][1,1'-联苯]-4-胺、萘-1,2-二胺和联苯胺等。在MFC还原偶氮染料中,萘环和苯环被认为是最常见的降解产物。在本研究中,乙酸盐氧化和硫化物氧化对产生电子起着重要作用,而偶氮染料则接受电子来破坏刚果红中的偶氮键。有研究[17]表明,3,4-二氨基萘-1-磺酸和联苯胺为刚果红降解的主要产物,与本研究结果一致。值得注意的是,MFC中染料降解的终产物主要是苯环和萘环,为了避免终产物对水体环境的污染,在污水排入自然环境之前,还需要进行进一步的好氧生物处理。
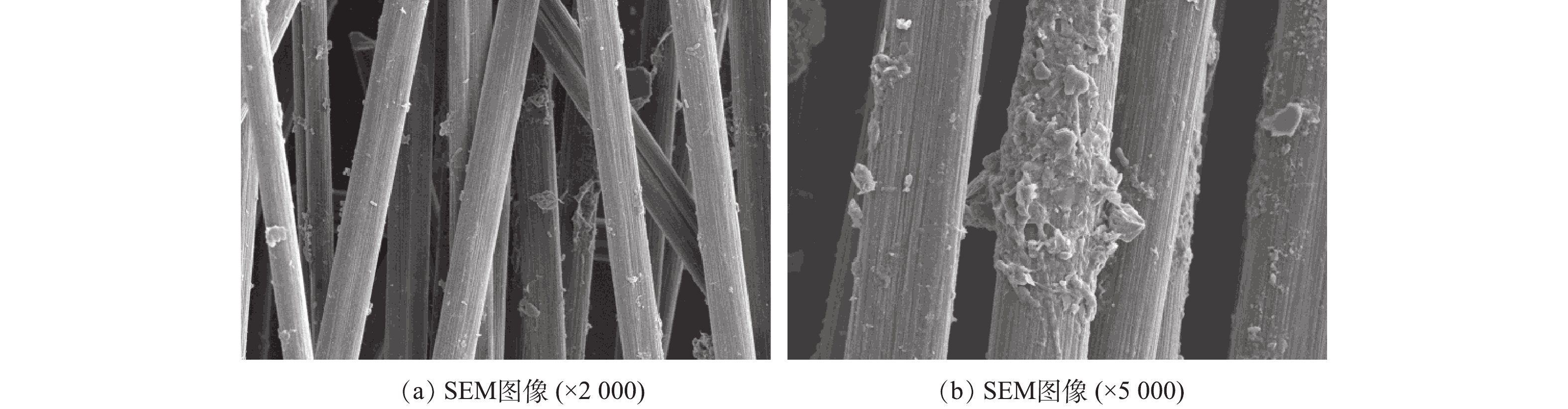
在MFC中硫化物氧化期间每4 h测定不同种类的硫物质(硫化物、硫酸盐、硫代硫酸盐和亚硫酸盐)的浓度。当硫酸盐(
$ {\rm{SO}}_{\rm{4}}^{{\rm{2 - }}}$ )和亚硫酸盐($ {\rm{SO}}_{\rm{3}}^{{\rm{2 - }}}$ )的浓度低于2 mg·L−1时可忽略不计。反应器中的总溶解硫化物浓度约为150 mg·L−1。在MFC中均检测到可溶性硫化物、单质硫、硫代硫酸盐和硫酸盐,表明硫化物可通过自发电化学反应实现单质硫、硫代硫酸盐和硫酸盐的转化。DAHL[18]的研究表明,通常在硫化物(HS−)的氧化过程中,首先会在黄素细胞色素c-硫化物脱氢酶(flavocytochrome c sulfide dehydrogenase,FCSD)和硫醌氧化还原酶(sulfide-quinone reductase,SQR)的作用下生成硫单质(S0),然后将电子分别转移到膜结合的细胞色素池或醌池中。随着氧化反应的进行,硫酸盐和硫代硫酸盐浓度逐渐增加,分别在36 h后达到45.41 mg·L−1和70.31 mg·L−1。在最后4 h可以观察到硫酸盐生成速率急剧增加,而硫化物浓度有所降低,这表明硫化物(S2−)首先被氧化成单质硫(S0),之后进一步氧化成其他更高价态(硫酸盐和硫代硫酸盐)。然而,未从系统中检测到亚硫酸盐($ {\rm{SO}}_{\rm{3}}^{{\rm{2 - }}}$ ),离子色谱未出现特征峰面积,与MIN等[19]的研究结果相一致。由图9可见,在生物膜中发现了一定数量的圆形菌毛样组分,表明与裸阳极相比,阳极生物膜密集附着有各种形状的微生物,这些微生物有利于硫化物氧化和电子转移。由图9(a)可以清楚地观察到被白色沉淀物覆盖的阳极电极。EDX分析结果表明,沉淀物中含有大量的单质硫(S0),作为硫化物氧化反应中的中间产物,硫化物可以与单质硫同时结合形成多硫化物($ {\rm{S}}_{{n}}^{{\rm{2 - }}}$ ),这在正常情况下发生率很高[20]。硫在不同pH条件下的存在形式有很大区别,S2−、H+和H2S之间的动态反应平衡方程如式(4)~式(7)所示。
当pH=7.0时,将[H+]=10−7代入式(7),则[S2−]/[H2S]=9.23×10−7。这意味着,当pH=7.0时,硫以H2S的形式存在,H2S可以通过空气阴极膜(如碳布)扩散,或取样时H2S气体立即从反应器中逸出。pH<7.0时H2S含量较高,酸性和偏碱性的条件下,HS−的含量增加,有利于硫化物向硫单质及硫酸盐的生成;而pH>7.0时S2−含量较高,硫酸盐含量减少。在高碱性条件下,单质硫可能是硫化物不完全氧化的主要产物,KRISHNAKUMAR等[21]也发现了缺氧条件下硫化物氧化的相似行为。许多研究表明,高负荷和高碱性pH条件下硫化物氧化可能导致过渡产物的积累,如硫代硫酸盐和多硫化物,导致硫化物去除效率较低,说明H+在电化学反应和微生物催化反应中起主导作用。由此可得出,在中性和偏碱性的条件下硫化物的降解速率是最快的,与本文的结论相符。同时,对于大多数细菌来说,细菌生长的pH为4.0~9.0,而其生长的最佳pH为6.5~7.5。KRISHNAKUMAR等[12]的研究表明,当反应器的pH控制在8.0时,达到了最大硫化物负载和硫回收率。在本研究中,当pH=7.0时,可获得最高的硫化物氧化率和染料脱色率。
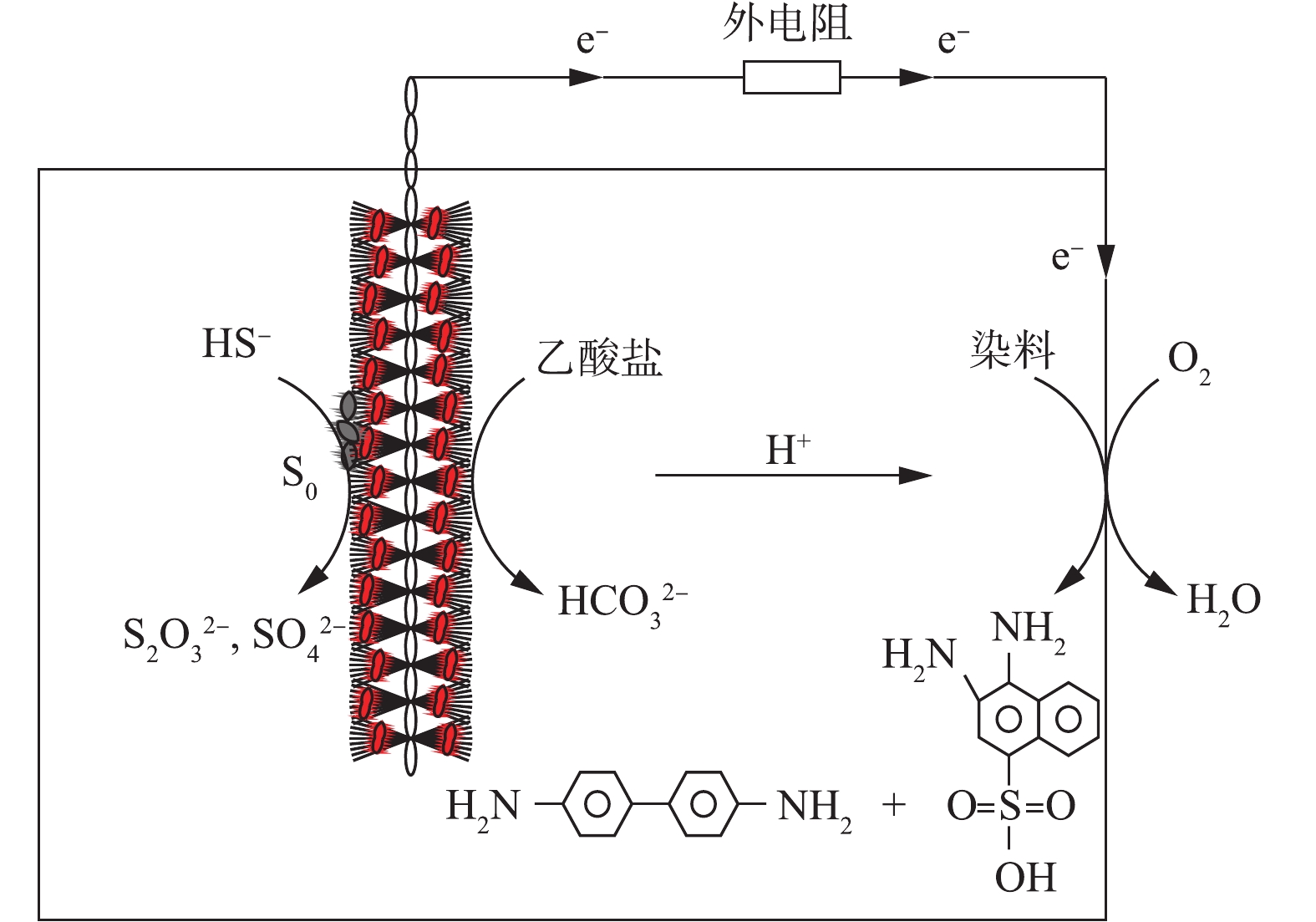
综上,单室空气阴极微生物燃料电池可以实现在硫化物和偶氮染料去除的同时产生电能。其反应机理如图10所示,硫化物以其强还原性和电子转移性加速偶氮染料的脱色。乙酸盐作为微生物的碳源,在SRB和产电微生物的作用下被氧化为
$ {\rm{HCO}}_{\rm{3}}^{{\rm{2 - }}}$ 和H+。硫化物一方面可以借助阳极生物膜上或阳极液中的SOB直接被氧化为硫酸盐和硫代硫酸盐等;另一方面硫化物可先被硫氧化菌氧化被硫单质,然后硫单质一部分黏附在阳极碳刷上,另一部分悬浮在阳极液中,其中的部分硫单质在SOB的作用下发生进一步的氧化作用,被氧化为$ {\rm{SO}}_{\rm{4}}^{{\rm{2 - }}}$ 和$ {{\rm{S}}_{\rm{2}}}{\rm{O}}_{\rm{3}}^{{\rm{2 - }}}$ 等含硫物质。本研究中S0、$ {\rm{SO}}_{\rm{4}}^{{\rm{2 - }}}$ 、$ {{\rm{S}}_{\rm{2}}}{\rm{O}}_{\rm{3}}^{{\rm{2 - }}}$ 的产生可能得益于SOB,SOB加速了MFC中物质的运输和代谢。硫化物在电化学活性和微生物作用下产生电子和质子,偶氮染料得到电子,生成芳香族类无色产物。实验证明刚果红的降解产物为4-氨基-1-萘磺酸钠,联苯胺和3,4-二氨基萘-1-磺酸。其中一些电子和质子分别通过外部电路和溶液到达阴极,与氧气结合生成水。因此,由于硫化物的强还原性、电子转移性和SOB富集,能够显著地加速偶氮染料的降解速率。
2.1. pH对MFC产电性能的影响
2.2. pH对硫化物和偶氮染料去除的影响
2.3. 不同pH条件下CV分析
2.4. 硫的转化及刚果红降解中间产物分析
-
1) pH对MFC系统的产电性能具有一定影响。在中性条件下,MFC输出电压和最大功率密度最大,内阻最小,偏酸或偏碱的条件下MFC的产电性能均不同程度降低。
2)单室空气阴极MFC能够实现含硫偶氮染料废水的有效去除,pH对MFC系统的污染物去除具有一定影响。当pH=7.0时,刚果红、硫化物和COD的去除率均达到最大值,分别为84.60%、98.40%和49.56%。而过酸或过碱的条件下,产电菌的活性会显著降低,阳极反应速率减慢。MFC的功率密度较低是制约MFC扩大化的主要因素。
3) pH可影响阳极产电菌的氧化还原能力,在中性条件下阳极产电菌的氧化能力最强。UV-vis和LC-MS分析得出联苯胺和3,4-二氨基萘-1-磺酸为刚果红降解反应的主要中间产物。



 下载:
下载: